Brandon Easparro¹, Claire McClain¹, Steven Cupello¹, Sara Amirahmadi¹, Tesfaye Kemsi¹, Jeff Roeder¹, and Travis Butts¹
- Omega Bio-tek, Inc., Norcross, GA 30071
Introduction
Plasma is a well-known sample type used in the isolation of cell-free DNA (cfDNA) for disease detection, diagnosis, and monitoring. cfDNA consists of small fragments of DNA released by cells as a result of processes such as apotosis, damage to cells, and the shedding of DNA by normal cells. Leveraging the information stored in cfDNA can revolutionize diagnostics, enabling tests to track cancer progression, assess treatment responses, and customize personalized therapies. Omega Bio-tek has developed a semi-automated solution to extract cfDNA from 4 mL of human-derived plasma with the MB Fit24™ cfDNA Kit (B3298-10-48PF) on the MagBinder® Fit24 with minimal manual intervention. The MagBinder® Fit24 (B1-001-24) is a nucleic acid purification system used together with prefilled reagent cartridges to extract DNA or RNA from up to 24 samples in a single run. Here, we demonstrate this workflow’s capability of extracting cfDNA from human-derived plasma compared to company T’s extraction workflow on their magnetic processor. cfDNA size, quality, and qPCR analyses were performed.
Materials and Methods
Pooled human-derived plasma was obtained from two different manufacturers: Innovative Research (Lot #42552) and Lampire Biological Laboratories (Lot #HMN1095120). cfDNA extraction was performed on 4 mL plasma (n=3) from each lot using the MB Fit24™ cfDNA Kit on the MagBinder® Fit24 and Company T’s cfDNA extraction kit on their equivalent magnetic processor. cfDNA size and quality were evaluated using Agilent’s TapeStation® 2200.
To evaluate the suitability of the purified DNA for downstream applications, real-time PCR was performed. cfDNA extracted from both lots of plasma using both the MB Fit24™ cfDNA kit and Company T’s cfDNA extraction kit was subjected to real-time PCR using primers for the reference gene RPI13a. Average Ct values were determined.
Learn more about the MagBinder® Fit24.
Results and Discussion
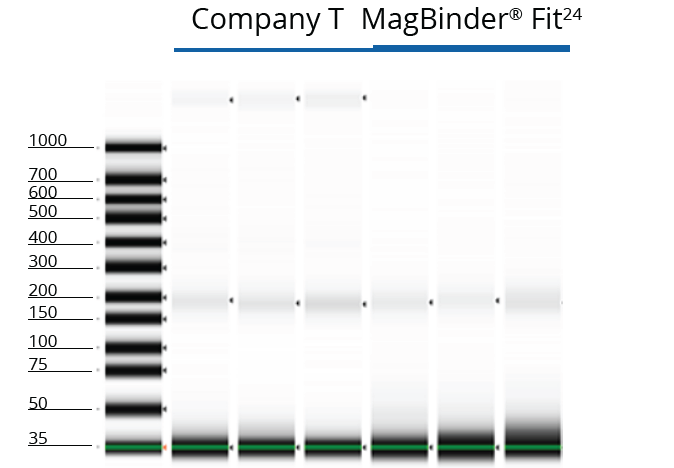
TapeStation Analysis of Purified cfDNA from 4 mL Innovative Plasma Samples
TapeStation Analysis of Purified cfDNA from 4 mL Lampire Plasma Samples
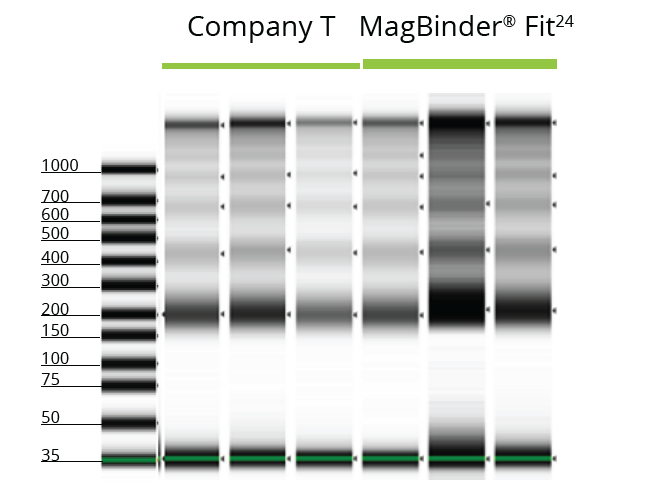
cfDNA extracted from 4 mL of human-derived plasma using kits from Omega Bio-tek and Company T was analyzed on the TapeStation 2200 to measure the size and integrity of the eluted cfDNA from both lots of plasma. The results of the TapeStation analyses are shown in Figures 1 and 2 and indicate that Omega Bio-tek’s MB Fit24™ cfDNA Kit can extract very small fragments of cfDNA with minimal genomic DNA contamination. In contrast, Company T’s eluate in Figure 1 contained high molecular weight fragments, indicating the presence of genomic DNA in the circulating DNA isolation.
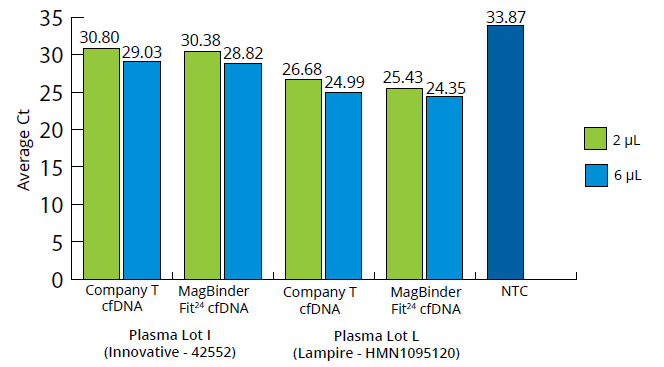
To establish the suitability of the cfDNA isolated from both lots of human-derived plasma for a variety of downstream applications, real-time PCR was performed using primers for the reference gene RPI13a. The average Ct values of the extracted cfDNA are shown in Figure 3. The averages obtained using the MB Fit24™ cfDNA Kit indicate positive amplification and efficiency, while Company T has slightly higher Ct values, indicating inhibition.
Average Ct Values from Purified cfDNA
Conclusions
Omega Bio-tek’s MB Fit24™ cfDNA Kit performed on the MagBinder® Fit24 offers an automated purification solution for cfDNA purifcation from plasma with minimal manual intervention. TapeStation analysis shows that cfDNA extracted with Omega Bio-tek’s Kit contained minimal genomic DNA contamination and inhibition. The size and integrity of the extracted cfDNA substantiate its ability in downstream applications such as PCR and NGS.
Implementing the MB Fit24™ cfDNA Kit on the MagBinder® Fit24 allows the user to run 24 samples in approximately 90 minutes with minimal offline preparation or manual intervention.
WP-0053