E.Z.N.A.® Gel Extraction Kit (V-spin)
$0.00 – $285.50
- Rapid – DNA recovery from an agarose gel in 15 min
- Specialized buffer system – Visual determination of optimum DNA binding for higher yields
- Safe – No phenol/chloroform extractions
- Versatile – Spin and vacuum formats available
- High-quality – DNA is suitable for a variety of downstream applications
The E.Z.N.A. Gel Extraction Kit uses spin-column technology to purify DNA fragments ranging from 100 bp to 10 kb from all grades of agarose gels with high recovery (> 80%). The kit uses a specialized binding buffer system that not only dissolves the gel slice and binds to the spin column but also includes a pH indicator for a visual representation of optimal pH for DNA binding. The bind step is followed by three rapid wash steps, and DNA is eluted with deionized water or elution buffer. Purified DNA is ready for a variety of downstream applications such as ligations, PCR amplification, restriction enzyme digestion, cloning, and various labeling reactions.
For Research Use Only. Not for use in diagnostic procedures.
| FEATURES | SPECIFICATIONS |
|---|---|
| Downstream application | Cloning, In Vitro Transcription, Nucleic Acid Labeling, PCR, Real-Time Quantitative PCR (qPCR), Sequencing, Southern Blotting |
| Elution volume | 30-50 µL |
| Starting material | Agarose gel slice |
| Starting amount | Up to 25 µg DNA |
| DNA recovered | >85% recovery, 70 bp to 20 kb |
| Processing mode | Manual, centrifugation/vacuum |
| Throughput | 1-12 |
| DNA binding technology | Silica mini spin column |
| Binding capacity | 25 μg |
| Processing time | <10 minutes |
| ITEM | AVAILABLE SEPARATELY |
|---|---|
| HiBind® DNA Mini Columns | View Product |
| 2 mL Collection Tubes | View Product |
| XP2 Binding Buffer | View Product |
| Elution Buffer | View Product |
| SPW Buffer |
Electrophoretic Analysis
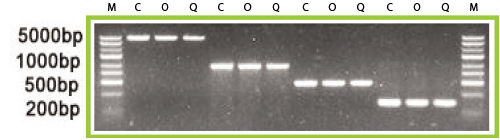
Figure 1. DNA ladder was run on a 2% agarose gel and 4 different fragment sizes (200 bp, 500 bp, 1 kb, and 5 kb) were recovered using Omega Bio-tek’s E.Z.N.A.® Gel Extraction Kit and a comparable kit from Company Q following manufacturer’s recommended protocols. DNA was analyzed on a 2% TBE agarose gel with the respective companies eluate being compared to the original amount used in the gel extraction procedure. M: size marker; C: control; O: Omega Bio-tek; Q: company Q.
DNA Fragment Recovery Comparison
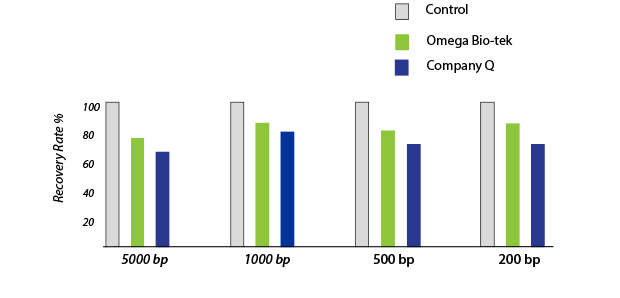
Figure 2. DNA was purified from an agarose gel using Omega Bio-tek’s E.Z.N.A.® Gel Extraction Kit and a comparable kit from Company Q. The concentration of the recovered DNA was determined by optical density measurements using Thermo Scientific’s NanoDrop™ 2000c system. The purified DNA normalized to input amount is shown above.
FAQs
How can I improve recovery and get higher A260/A230 ratios using the E.Z.N.A. Gel Extraction Kit?
Few recommendations:
- Use running buffers made fresh. (Reusing the same running buffer messes with the pH of binding and, in fact, inhibits DNA binding to the column).
- Double the binding buffer (XP2) to dissolve the gel pieces if the gel pieces are hard to dissolve. You would have to run the DNA/agarose solution through the column more than once in this case. Instead of heating in step 5, pipette the solution up and down until the agarose is completely dissolved. This results in a higher recovery percentage with larger fragments in our experiments.
- After the Gel/Binding Buffer mixture has completely dissolved, monitor the color. If the color of the mixture becomes orange or red, add 5 µL 5M sodium acetate (pH 5.2) to bring the pH down. After this adjustment, the color of the Gel/Binding Buffer mixture should be light yellow. It needs to be yellow for good binding onto the column (most times, reusing running buffers messes up the binding pH and hence recovery).
- Heat the elution buffer to 70 °C and add to the column directly. Incubate on the column for 5 minutes and perform a second elution step. That is, pipette the first eluate back on the column and spin it down.
Low A260/A230 ratios alludes to salt carryover (guanidine in the binding buffer is not completely washed off during wash steps). Typically increasing the wash steps improves this ratio – so instead of 2 SPW wash steps, do 3.
Can the E.Z.N.A. Gel Extraction Kit be used for PCR clean-up as well?
The E.Z.N.A. Gel Extraction Kit (D2500) can be used for both PCR clean-up and gel extraction.
What is the binding capacity of the column in D2500?
Our HiBind® DNA Mini Columns can bind DNA between 100 bp – 10 kbp of DNA and the binding capacity of the column is 100 µg.
Product Information
Safety Data Sheets
| Components | Hazard Standards | Languages | Link |
|---|---|---|---|
| Elution Buffer | GHS | Spanish | |
| Elution Buffer | REACH | Greek | |
| Elution Buffer | GHS | English | |
| Elution Buffer | REACH | Portuguese | |
| Elution Buffer | REACH | Hungarian | |
| Elution Buffer | REACH | Dutch | |
| Elution Buffer | WHMS | French | |
| Elution Buffer | WHMS | English | |
| Elution Buffer | REACH | Swedish | |
| Elution Buffer | REACH | Spanish | |
| Elution Buffer | REACH | Norwegian | |
| Elution Buffer | REACH | Italian | |
| Elution Buffer | REACH | German | |
| Elution Buffer | REACH | French | |
| Elution Buffer | REACH | Finnish | |
| Elution Buffer | REACH | Danish | |
| Elution Buffer | REACH | English | |
| SPW Buffer | REACH | Greek | |
| SPW Buffer | REACH | Finnish | |
| SPW Buffer | WHMS | French | |
| SPW Buffer | WHMS | English | |
| SPW Buffer | GHS | Spanish | |
| SPW Buffer | GHS | English | |
| SPW Buffer | REACH | Swedish | |
| SPW Buffer | REACH | Spanish |