Introduction
Circulating, cell-free DNA (cfDNA) holds great clinical significance for non-invasive disease detection, diagnosis, and monitoring. cfDNA are usually small fragments of DNA (size distribution peak at ~170 bp) found circulating in plasma, serum, or other bodily fluids and offers tremendous potential as a screening method for tumors, cancer, as well as in fetal DNA studies. Circulating DNA extraction is often challenging as they are found in low quantities and accurate, consistent methods are needed to isolate the less abundant cfDNA with higher sensitivity. In this context, Omega Bio-tek has developed cfDNA extraction kits for manual processing using silica spin columns (E.Z.N.A.® Circulating DNA Kit, D3091) and for automated, high throughput workflows using magnetic beads (Mag-BIND® cfDNA Kit, M3298). Omega Bio-tek’s kits allow flexible sample inputs (1-4 mL for column-based; 0.5-4 mL for magnetic bead-based) and low elution volumes for isolating concentrated cfDNA suitable for a variety of downstream applications such as qPCR, next-generation sequencing, etc. In this study, we elucidate the key features of the Omega Bio-tek circulating DNA kits and compare their performance to that of Company Q’s column-based cfDNA kit in terms of DNA and quality.
Learn more about the Mag-Bind® cfDNA Kit.

Materials & Methods
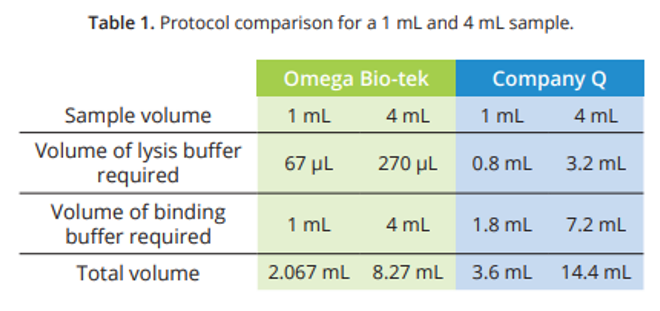
Circulating, cell-free DNA was extracted from 1 mL of unspiked serum using Omega Bio-tek’s Mag-BIND® cfDNA Kit and a comparable column-based kit from Company Q following manufacturer’s recommended protocols. The extraction workflow using the Mag-BIND® cfDNA Kit is outlined in Figure 1. The Omega protocol features reduced lysis and binding buffer volumes compared to Company Q (Table 1) making it automation-friendly not only for larger input sample volumes, but also for rapid processing of ninety-six 1 mL samples in a 96-well deep well plate. The DNA isolations were performed in triplicate and eluted in 60 µL volume. cfDNA purified from all the experiments was analyzed on Agilent’s TapeStation® 2200 to assess its fraction relative to the total DNA extracted.
Preparing high quality libraries is a critical component for the success of any next-generation sequencing application. In order to ensure the integrity of the purified cfDNA and to test its downstream application suitability, we prepared next-generation sequencing libraries using KAPA Biosystems’ HyperPrep Kit following the recommended protocol.Same number of PCR cycles was used for cfDNA extracted from both the Omega Bio-tek kit and the Company Q kit. Amplified DNA was analyzed on Agilent’s TapeStation® 2200 following library construction.
Results & Discussion
Figure 2 shows the electropherogram overlay of 1 mL unspiked serum sample purified using the Mag-BIND® cfDNA Kit and a comparable kit from Company Q. The TapeStation® results indicate that the Mag-BIND® cfDNA Kit could capture the circulating, cell-free DNA with little to no genomic DNA contamination. In contrast, Company Q’s eluate contained high molecular weight fragments indicating the presence of genomic DNA in the circulating DNA isolation.
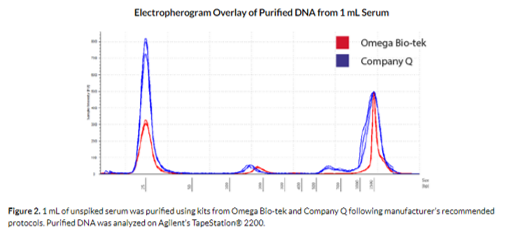
To arrive at the cfDNA concentration without any interference from the genomic DNA, we utilized the regional analysis functionality of the TapeStation® 2200 Analysis Software. The DNA concentration within the 100-300 bp region where cfDNA is most likely to be present was quantified using the software and show in Table 2. The cfDNA extracted from the Mag-BIND® cfDNA Kit within the 100-300 bp region was determined to be 45.2 pg/µL and was significantly higher than that of Company Q’s at 25.1 pg/µL (Table 2).

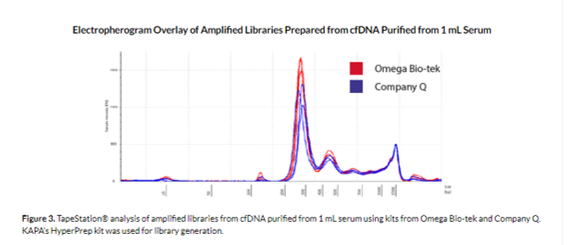
Using the cfDNA extracted from the Mag-BIND® cfDNA Kit and Company Q kits as the input, we could successfully generate libraries using KAPA’s HyperPrep kit and from the electropherogram overlay amplified libraries seem comparable (Figure 3). This data indicates that the purified DNA was of sufficiently high quality for fortifying its suitability for various downstream applications.
Conclusions
Omega Bio-tek’s circulating DNA kits offer a better solution to isolate the cfDNA with little or no genomic DNA contamination compared to the kit from Company Q. Results from the TapeStation® showed that only DNA around 170 bp was found, and high molecular weight DNA was not detected. We could successfully prepare libraries from low amounts of cfDNA as the input and demonstrated its suitability for NGS and other downstream applications.
For high throughput applications, Omega Bio-tek’s Mag-BIND® cfDNA Kit using magnetic bead-based purification technology can be automated on most open-ended liquid handling platforms and provides a solution for processing 192 samples (2 mL) in 2 hours from sample to tube to extracted DNA.


